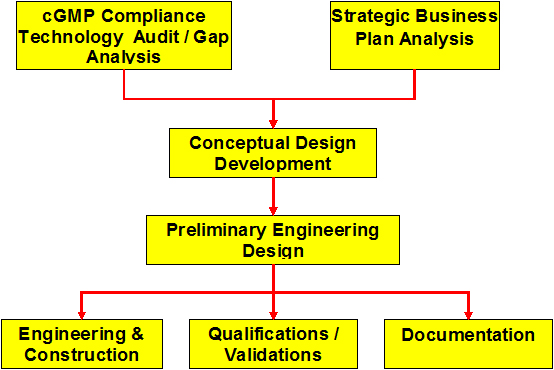
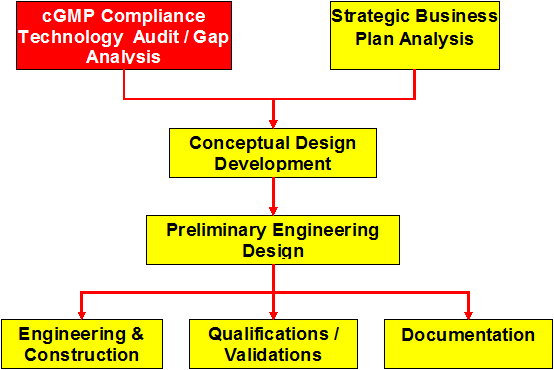
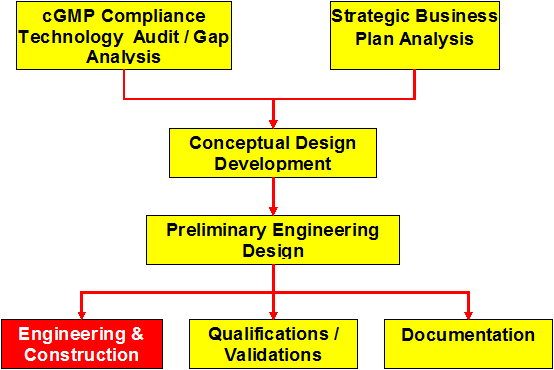
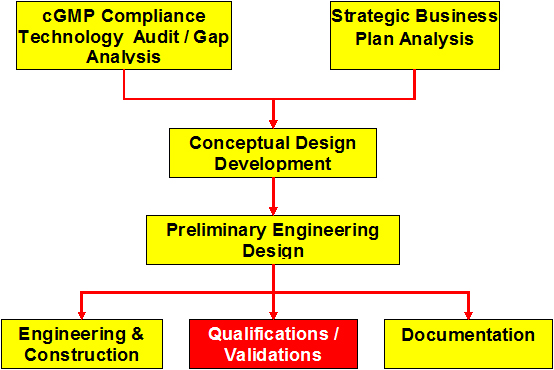
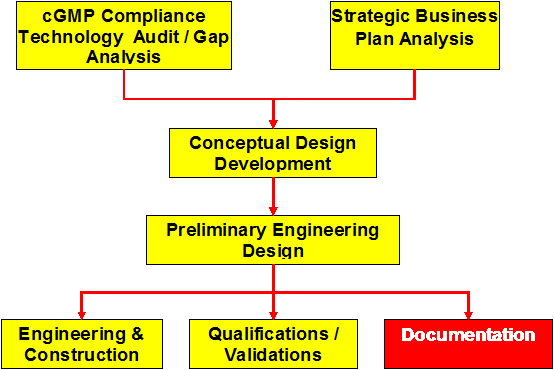
cGMP Compliance Technology Audit/Gap Analysis
- Equipment and Facilities
- Processes
- Quality Systems
- Documentation
- Organizational Structure
- Risk assessment
- Enable companies to determine which qualification/validation/facilities & equipment upgrades are necessary
- Provide support with the implementation of identified measures.
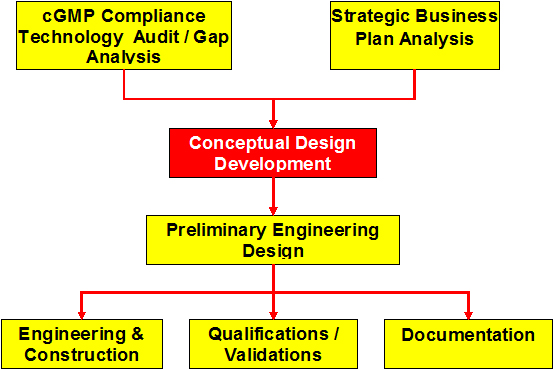
Development of the Conceptual Design
- Prerequisite and a guide to prepare technical requirements document (User Requirements Specifications) for economical and functional preparation and implementation of detailed engineering design project for facility and equipment upgrade/retrofit/new construction within the cGMP framework, based on the regulatory Risk assessment and strategic business development plan or analysis
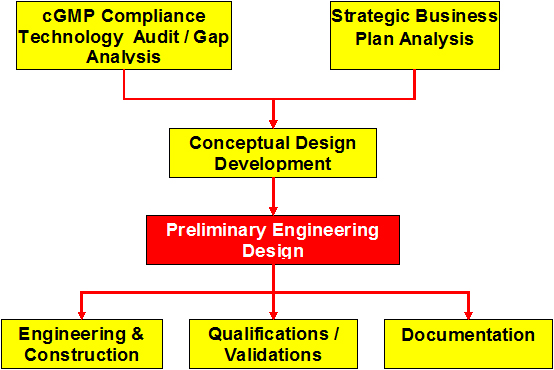
Preliminary Engineering Design
- Field survey to confirm the layout of architectural system components and mechanical, electrical and plumbing infrastructure.
- Preparation of material, personnel and equipment flow diagrams for all process areas.
- Selection and defining utility requirements and Client`s needs associated with new and existing processes and equipment. Preparation of the process equipment list for Client`s review and approval that will form the basis for budgetary equipment costs. Organization (optional) of pre-selection of vendors and a bid process for the equipment to include US, European, Asian and local suppliers.
- Preparation of Area Classification and Pressurization Plan drawings for each of the processing areas with reference to European Grade levels as appropriate.
- Preparation room air change rate calculations and development of preliminary HVAC Zoning plans so that the assessments of existing and sizing of new HVAC equipment can be made.
- Recommendation of the affecting set points for room temperature, humidity and pressurization, as well as acceptable operating, alert and action limits and how these will be monitored and controlled.
- Preparation of a Validation Master Plan.
Engineering & Construction
- Field Project Management
- Design (Architectural, Civil, Structural, Mechanical, HVAC, Plumbing, Electrical, Process)
- Purchasing/Expediting
- Construction
- Validation
- Start-up / Commissioning
- Manufacturing/Processing Personnel Training
Qualifications/Validations
- Qualifications/Validations
- Basic concepts
- Master plans (a company specific qualification/validation concept)
- Project specific master plan (GMP, Validation, SOPís)†
- Qualification (DQ/IQ/OQ/PQ)
- Development and preparation of all qualification protocols and relevant SOPís
- Execution of the protocols (at customer request) or helping a customer to execute
- Preparation of GMP compliant documentation describing the qualification activities for the customerís facility
- Validation
- Full technical and manpower support for preparation of protocols and reports and execution activities (although the latter is typically done by the manufacturer).
- Scope Ė process, cleaning, analytical methods, computerized systems
- Preparation of all necessary validation SOPís carefully adapted to the customerís needs
Documentation
- Documentation
- Technical support and execution (per customer`s request) of all necessary documentation
- Scope - documentation concepts, SOP`s, production documentation (batch records, cleaning records, etc.), technical documentation (development reports)
- Documentation review
- Training
- Preparation for inspection
- Technical support and/or execution and follow-up services
- Scope - Self audits, Audits of suppliers, Customer audits, Regulatory audits
- Remediation (FDA 483`s, Warning Letters)
- Technical support in preparation or execution of responses to local and/or international regulatory agencies (for example, EIR, 483`s and Warning letters)
- Technical support in preparation and/or execution of the corrective and preventative action (CAPA) master plans
- Regulatory Submissions
- Technical support and/or execution and follow-up services
- Scope - DMF, COS, Comparability protocols, CMC section
- Training
- Design of the comprehensive training program to cover initial and ongoing training requirements
- Conducting general GMP and specific training sessions on continues basis